Safety
Radiation safety
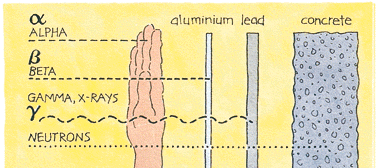
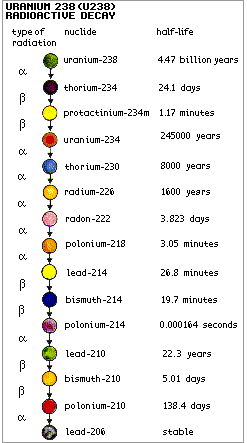
Ionising radiation and how it is measured - information from <a class="external" href="http://www.uic.com.au/neAp1.htm" rel="nofollow" target="_blank">Uranium Information Centre Ltd</a> Four kinds of nuclear radiation:
- Alpha particles: These are particles (atomic nuclei) consisting of 2 protons and 2 neutrons. They are intensely ionising but can be readily stopped by a few centimetres of air, a sheet of paper, or the human skin. They are only dangerous to people if they are released inside the body. Alpha-radioactive substances are safe if kept in any containers sealed to air.
- Beta particles: These are either electrons or positrons (therefore of very low mass). They can be stopped by a thin piece of wood or plastic and are generally less dangerous to people than gamma radiation. Exposure produces an effect like sunburn, but which is slower to heal. Beta-radioactive substances are also safe if kept in appropriate sealed containers.
- Gamma rays: These are high energy beams almost identical with x-rays and of shorter wavelength than ultraviolet radiation. They are very penetrating, and need substantial thicknesses of heavy materials such as lead, steel or concrete to shield them. They are the main hazard to people in dealing with sealed radioactive materials. Doses can be detected by the small badges worn by workers handling any radioactive materials. Gamma activity in a substance (e.g. rock) can be measured with a scintillometer or geiger counter.
- Neutrons: These are mostly released by nuclear fission, and apart from a little cosmic radiation they are seldom encountered outside the core of a nuclear reactor. Fast neutrons are very penetrating as well as (indirectly) being strongly ionising and hence very destructive to human tissue. They can be slowed down (or "moderated") by wood, plastic, or (more commonly) by graphite or water.NB: (X-rays are also ionising radiation, virtually identical to gamma rays, but not nuclear in origin.)
Radiation units:
- The amount of ionising radiation absorbed in tissue can be expressed in grays, 1 Gy = 1 J/kg. However, since neutrons and alpha particles cause more damage per gray than gamma or beta radiation, another unit, the sievert
(Sv) is used in setting radiological protection standards. - One gray of beta or gamma radiation has one sievert of biological effect, one gray of alpha particles has 20 Sv effect and one gray of neutrons is equivalent to around 10 Sv (depending on their energy).
- Total dose is thus measured in sieverts (or millisieverts - mSv - one thousandth of a sievert, or microsieverts - µSv - one millionth of a sievert).
- The rate of dose is measured in milli or micro sieverts per hour or year. For instance, our natural dose is around 2 mSv/yr, and maximum annual dose allowed for a uranium miner is 20 mSv/yr, though that received in Australian and Canadian mining operations is typically less than half of this. (These levels contrast with those which are harmful in a disaster scenario: with gamma radiation a short term dose of 1 Sv causes (temporary) radiation sickness, 5 Sv would kill about half the people receiving it in a month and a burst of 10 Sv would be fatal to all in a matter of days.
- The 28 radiation fatalities at Chernobyl appear to have received more than 5 Sv in a few days, those suffering acute radiation sickness averaged 3.4 Sv.)
- The becquerel (Bq) is the unit or a measure of actual radioactivity in material (as distinct from the radiation it emits, or the human dose from that), with reference to the number of nuclear disintegrations per second (1 Bq = 1 disintegration/sec). Quantities of radioactive material are commonly estimated by measuring the amount of intrinsic radioactivity in becquerel - one Bq of radioactive material is that amount which has an average of one disintegration per second, ie an activity of 1 Bq.
- Older units of radiation measurement continue in use in some literature:
1 gray = 100 rads
1 sievert = 100 rem
1 becquerel = 27 picocuries or 2.7 x 10-11 curies One curie was originally the activity of one gram of radium-226, and represents 3.7 x 1010 disintegrations per second (Bq).
I<a class="external" href="http://www.uic.com.au/ral.htm" rel="nofollow" target="_blank">nformation below from Radiation and life</a>
<a class="external" href="http://www.uic.com.au/ral.htm" rel="nofollow" target="_blank">
</a>
Absorbed dose
is a measure of the energy deposited in a medium by ionizing radiation. It is equal to the energy deposited per unit mass of medium, and so has the unit J/kg or gray (Gy) where 1Gy = 1Jkg-1. The absorbed dose is not a good indicator of the likely biological effect. 1 Gy of alpha radiation would be much more biologically damaging than 1 Gy of photon radiation for example. Appropriate weighting factors can be applied reflecting the different relative biological effects to find the equivalent dose. The risk of stochastic effects due to radiation exposure can be quantified using the effective dose, which is a weighted average of the equivalent dose to each organ depending upon its radiosensitivity. Other related values include:
- absorbed dose rate (Gy.s-1): amount of radiation delivered over a time period
- rad: the international unit of absorbed dose pre-1980; 1Gy = 100rad
- kerma (Gy): kinetic energy released to matter
The equivalent dose
(HT) is a measure of the radiation dose to tissue where an attempt has been made to allow for the different relative biological effects of different types of ionizing radiation. In quantitative terms, equivalent dose is less fundamental than absorbed dose, but it is more biologically significant. Equivalent dose has units of sieverts.
Equivalent dose (HT) is calculated by multiplying the absorbed dose to the organ or tissue (DT) with the radiation weighting factor, wR. This factor is dependant on the type and energy of the incident radiation. The value of wR is 1 for x-rays, gamma rays and beta particles, but higher for protons, neutrons, alpha particles etc.Stochastic effects
Stochastic effects in occur by chance and can be compared to deterministic effects which result in a direct effect.
Cancer induction as a result of exposure to radiation occurs in a stochastic manner: there is no threshold point and risk increases in a linear fashion with dose. This is known as the linear no threshold theory.
Although the risk increases with dose, the severity of the effects do not; the patient will either develop cancer or they will notDeterministic effects
describe a cause an effect relationship between radiation and some side-effects. They are also called non-stochastic effects to contrast their relationship with the chance-like stochastic effects, e.g. of cancer induction. Deterministic effects have a threshold below which, the effect does not occur. The threshold may be very small and may vary from person to person. However, once the threshold has been exceeded, the liklihood of an effect occuring increases rapidly with dose until a dose-level is reached at which the effect will invariably occur. Examples of deterministic effects (doses are given as absorbed dose):
- skin erythema: 2-5Gy
- irreversible skin damage: 20-40Gy
- hair loss: 2-5Gy
- sterility 2-3Gy
- cataracts: 5Gy
- lethality (whole body): 3-5Gy
- fetal abnormality: 0.1-0.5Gy
HOW MUCH IONISING RADIATION IS DANGEROUS? Radiation levels and their effects
The following gives an indication of the likely effects of a range of whole body radiation doses and dose rates to individuals:
10,000 mSv (10 sieverts) as a short-term and whole-body dose would cause immediate illness, such as nausea and decreased white blood cell count, and subsequent death within a few weeks.Between 2 and 10 sieverts in a short-term dose would cause severe radiation sickness with increasing likelihood that this would be fatal.
1,000 mSv (1 sievert) in a short term dose is about the threshold for causing immediate radiation sickness in a person of average physical attributes, but would be unlikely to cause death. Above 1000 mSv, severity of illness increases with dose.
If doses greater than 1000 mSv occur over a long period they are less likely to have early health effects but they create a definite risk that cancer will develop many years later.
Above100 mSv, the probability of cancer (rather than the severity of illness) increases with dose. The estimated risk of fatal cancer is 5 of every 100 persons exposed to a dose of 1000 mSv (ie. if the normal incidence of fatal cancer were 25%, this dose would increase it to 30%).
50 mSv is, conservatively, the lowest dose at which there is any evidence of cancer being caused in adults. It is also the highest dose which is allowed by regulation in any one year of occupational exposure. Dose rates greater than 50 mSv/yr arise from natural background levels in several parts of the world but do not cause any discernible harm to local populations.
20 mSv/yr averaged over 5 years is the limit for radiological personnel such as employees in the nuclear industry, uranium or mineral sands miners and hospital workers
10 mSv/yr is the maximum actual dose rate received by any Australian uranium miner.
-3-5 mSv/yr is the typical dose rate (above background) received by uranium miners in Australia and Canada
3 mSv/yr (approx) is the typical background radiation from natural sources in North America, including an average of almost 2 mSv/yr from radon in air.
2 mSv/yr (approx) is the typical background radiation from natural sources, including an average of 0.7 mSv/yr from radon in air. This is close to the minimum dose received by all humans anywhere on Earth.
0.3-0.6 mSv/yr is a typical range of dose rates from artificial sources of radiation, mostly medical
0.05 mSv/yr, a very small fraction of natural background radiation, is the design target for maximum radiation at the perimeter fence of a nuclear electricity generating station. In practice the actual dose is less
Radiation safety Links
- <a class="external" href="http://www.arpansa.gov.au/basics/" rel="nofollow" target="_blank">Radiation Basics (ARPANSA)</a>
- <a class="external" href="http://en.wikipedia.org/wiki/Ionizing_radiation" rel="nofollow" target="_blank">Ionizing Radiation article on Wikipedia Background Radiation article on wikiped</a>
- <a class="external" href="http://en.wikipedia.org/wiki/Radiation_hormesis" rel="nofollow" target="_blank">Is a little Radiation good for you ? see article on Radiation hormesis on wikipedia </a>
- <a class="external" href="http://www.radiologyinfo.org/en/safety/index.cfm?pg=sfty_xray&bhcp=1" rel="nofollow" target="_blank">Radiation Exposure in X-ray Examinations</a>
- <a class="external" href="http://www.arpansa.gov.au/" rel="nofollow" target="_blank">The Australian Radiation Protection and Nuclear Safety Agency (ARPANSA)</a>
- <a class="external" href="http://www.environment.sa.gov.au/epa/radiation.html" rel="nofollow" target="_blank">EPA Radiation Safety (Radiation and Health Info)</a>
- <a class="external" href="http://www.iaea.org/" rel="nofollow" target="_blank">International Atomic Energy Commission</a>
- <a class="external" href="http://www.icrp.org/" rel="nofollow" target="_blank">The International Commission on Radiological Protection</a>
International and National Organisations<a class="external" href="http://irpa.sfrp.asso.fr/" rel="nofollow" target="_blank"></a>
- <a class="external" href="http://irpa.sfrp.asso.fr/" rel="nofollow" target="_blank">International Radiation Protection Association</a> (IRPA)<a class="external" href="http://www.iaea.or.at/" rel="nofollow" target="_blank">
</a> - <a class="external" href="http://www.iaea.or.at/" rel="nofollow" target="_blank">International Atomic Energy Agency</a> (IAEA)
- <a class="external" href="http://www.icrp.org/" rel="nofollow" target="_blank">International Commission on Radiological Protection</a> (ICRP)<a class="external" href="http://www.sz.shuttle.de/dm1001/icnirp.htm" rel="nofollow" target="_blank">
</a> - <a class="external" href="http://www.sz.shuttle.de/dm1001/icnirp.htm" rel="nofollow" target="_blank">International Commission on Non-Ionizing Radiation Protection</a> (ICNIRP)<a class="external" href="http://www.icru.org/" rel="nofollow" target="_blank">
</a> - <a class="external" href="http://www.icru.org/" rel="nofollow" target="_blank">International Commission on Radiation Units and Measurements</a> (ICRU)
- <a class="external" href="http://www.health.gov.au/arl/" rel="nofollow" target="_blank">Australian Radiation Laboratory</a> (ARL)
- <a class="external" href="http://www.nrl.moh.govt.nz/" rel="nofollow" target="_blank">New Zealand National Radiation Laboratory</a> (NRL)
- <a class="external" href="http://www.ansto.gov.au/" rel="nofollow" target="_blank">Australian Nuclear Science and Technology Organisation</a> (ANSTO)
- <a class="external" href="http://hna.ffh.vic.gov.au/phb/hprot/rsu/rsu.html" rel="nofollow" target="_blank">Victorian Radiation Safety Unit</a>
- <a class="external" href="http://www.public.health.wa.gov.au/radiat.htm" rel="nofollow" target="_blank">Western Australia Radiation Health Section</a>
- <a class="external" href="http://www.epa.gov/radiation/" rel="nofollow" target="_blank">US EPA Radiation Protection Division </a>
- <a class="external" href="http://orvc.saic.com/nrc_rad.htm" rel="nofollow" target="_blank">US NRC Radiation Protection Information</a>
- <a class="external" href="http://www.nrc.gov/NRC/RG/index.html" rel="nofollow" target="_blank">US NRC Regulatory Guides</a>
- <a class="external" href="http://www.ncrp.com/" rel="nofollow" target="_blank">National Council on Radiation Protection and Measurements</a> (NCRP)
- <a class="external" href="http://www.nrpb.org.uk/" rel="nofollow" target="_blank">UK National Radiation Protection Board</a> (NRPB)
- <a class="external" href="http://www,srp-uk.org/" rel="nofollow" target="_blank">UK Society for Radiological Protection</a> (SRP)
- <a class="external" href="http://www.shef.ac.uk/%7Eaurpo/index.html" rel="nofollow" target="_blank">UK Association of University Radiation Protection Officers</a> (AURPO)
- <a class="external" href="http://www.hps.org/" rel="nofollow" target="_blank">US Health Physics Society</a> (HPS)
- <a class="external" href="http://www.radres.org/" rel="nofollow" target="_blank">Radiation Research Society</a> (RRS)
Other Sources
- <a class="external" href="http://www.umich.edu/%7Eradinfo/" rel="nofollow" target="_blank">Radiation and Health Physics </a>
- <a class="external" href="http://www.orau.gov/ehsd/ridic.htm" rel="nofollow" target="_blank">Radiation Internal Dose Information Center</a> (RIDIC)
- <a class="external" href="http://www.rerf.or.jp/eigo/experhp/rerfhome.htm" rel="nofollow" target="_blank">Radiation Effects Research Foundation</a> (RERF)
- <a class="external" href="http://http//www.physics.isu.edu/radinf/" rel="nofollow" target="_blank">The Radiation Information Network</a> (Idaho State University)
- <a class="external" href="http://ean.cepn.asso.fr/" rel="nofollow" target="_blank">European ALARA Network</a> (EAN)
- <a class="external" href="http://www.open.gov.uk/hse/hthdir/rad_prad/" rel="nofollow" target="_blank">The Radiation Protection Advisor</a> (Newsletter of the UK Health & Safety Executive)
- <a class="external" href="http://www.hc-sc.gc.ca/ehp/ehd/catalogue/rpb.htm" rel="nofollow" target="_blank">Health Canada Radiation Protection Publications</a>
- <a class="external" href="http://www.westnet.net.au/Walkabout/radlinks/index.html" rel="nofollow" target="_blank">Nick's World Collection of Radiation Links</a>
Societies in Australia and/or New Zealand
- <a class="external" href="http://xray.anu.edu.au/anzsnm/anzsnm.htm" rel="nofollow" target="_blank">Australian and New Zealand Society of Nuclear Medicine</a>